ARTICLES
MOELGAARD
CONSULTING

Quality by Design: How to Harvest the Benefits?
06/23/2016
Gert Moelgaard about Trends in the Pharmaceutical Industry
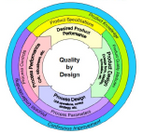
Quality by Design is decried as a theoretical, academic and complex system. In the meantime, it is set as a standard in the US pharmaceutical industry. In Europe, the concept is slowly accepted. Fact is: Development based on QbD will be safer, faster and more agile.
The term Quality by Design (QbD) is less than ten years old in the pharmaceutical industry but it gained a lot of attention especially from companies supplying the US market because.......

Trends der Pharmaproduktion
Das neue Quality Metrics-Konzept der FDA – So profitieren Sie davon!
Was verbirgt sich hinter dem Quality Metrics-Konzept der FDA? Die FDA denkt über neue Methoden zur Messung der Qualitätsperformance und -kultur nach. Was bedeutet das für die Verantwortlichen?

New Concept - New Chance
Quality Metrics in pharmaceutical manufacturing: Challenge or Opportunity?
By Gert Moelgaard
A new way of measuring quality performance and quality culture in pharmaceutical companies is on the horizon.
Are European companies ready for FDA´s Quality Metric Concept?

Small scale - special solutions
By Gert Moelgaard
Specialty medicines and facilities of the future are the new challenges. The biggest pharmaceutical products are now specialty medicines. Not 'biggest' from a volume perspective, but from a value perspective. This has significant implications for manufacturing, especially on flexibility needs.
The article was published in Process Worldwide, no. 6, November 2014, Volume 16. It is the fifth in a series of articles by Gert Mølgaard on the future of pharmaceutical production.
http://www.nnepharmaplan.com/Global/Downloads/Expert-articles/Small-scale-special-solutions-2014.pdf
Is Quality by Design a forgotten term?
By Gert Moelgaard
Quality by Design is a powerful instrument to make pharmaceutical production safe, robust and more efficient. But ten years after starting we are still at the beginning of the QbD era. What has to be done to make the concept a breakthrough?
The article was published in Process Worldwide, no. 1, Volume 17, March 2015. It it the sixth and last in a series of articles by Gert Mølgaard focused on the future of pharmaceutical production.
http://www.nnepharmaplan.com/Global/Downloads/Expert-articles/Small-scale-special-solutions-2014.pdf
Fast to market — how to face the upcoming challenges
By Gert Moelgaard
Pharmaceutical manufacturing operations must prepare for faster drug approvals and increased time-to-market pressure. Pharmaceutical companies, which aims to be best-in-class, will need to explore new ways to make the “facilities of the future". In this article, Gert Moelgaard elaborates on the future of pharmaceutical production.
The article was published in Process Worldwide, no. 1, March 2014, Volume 16. It is the first in a series of articles by Gert Moelgaard, Vice President for Strategic Development, focusing on the future of pharmaceutical production.
READ THE FULL ARTICLE.....
Breakthrough of continuous pharmaceutical manufacturing creates opportunities
By Gert Mølgaard
Over the last couple of years the term continuous manufacturing has become increasingly popular in pharmaceutical manufacturing. But it is still hard to answer the question: “Which efficiency benefits can be reached with continuous manufacturing in comparison to the batch procedure?”
The article was published in PROCESS Worldwide, vol. 17, issue 6, November 2015.
http://www.process-worldwide.com/breakthrough-of-continuous-pharmaceutical-manufacturing-creates-opportunities-a-512191/
GERMAN VERSION....
http://www.process.vogel.de/pharma/articles/511179/?cmp=beleg-mail
The future of medicine manufacture
By Gert Moelgaard
Economic pressures, new technology and the rise of biologics are all having a huge impact on the pharmaceutical industry. What will the next 10 years hold for drug and biologics manufacture? To answer that question, Gert Moelgaard reviews the factors driving big change in manufacturing and Guillaume Plane takes us on a tour of the facility of the near future.
The article was published in the Medicine Maker, issue 01, September 2014.
http://www.nnepharmaplan.com/Global/Downloads/Expert-articles/The-future-of-medicine-manufacture-2014.pdf
Process validation - preparing for the future
By Gert Moelgaard
In January 2014 the "new" FDA process validation guide celebrated its 3 years anniversary - and in February the European Commission launched EMA's draft to a new European Annex 15 for Validation and Qualification in the GMPs. However, although Annex 15 is only a draft, we already know enough to prepare our validation and qualification approach for the future. Actually some of the new practices are already becoming regulatory expectations - sometimes with significant impact on observations or even warning letters for pharmaceutical companies.
The article was published in Process Worldwide, no. 3, July 2014, Volume 16. It it the third in a series of articles by Gert Mølgaard focused on the future of pharmaceutical production.
http://www.nnepharmaplan.com/Global/Downloads/Expert-articles/Process-validation-preparing-for-the-future-2014.pdf

After the patent cliff - are you ready for change?
By Gert Moelgaard
After the patent cliff, pharmaceutical manufacturers have to face another challenge: product portfolios have changed from large to small volumes. There is a need for more flexibility and the question is: are pharmaceutical manufacturers ready for the requirements of “facilities of the future”.
The article was published in Process Worldwide, no. 5, October 2014, Volume 16. It is the fourth in a series of articles by Gert Mølgaard on the future of pharmaceutical production.
http://www.nnepharmaplan.com/Global/Downloads/Expert-articles/Afer-the-patent-cliff-ready-for-change-2014.pdf
The need for agile facilities in the future pharma reality
By Morten Munk
and Gert Moelgaard
This article elaborates on the possibilities of single-use technology and addresses how the biopharma production can benefit from the implementation of continuous manufacturing like other industries did before. These approaches play an important role in agile and flexible biopharma productions.
The article was published in the TechnoPharm magazine, issue 05, October 2015.
http://www.nnepharmaplan.com/Global/Downloads/Expert-articles/The-need-for-agile-facilities-in-the-future-pharma-reality-2015.pdf
Gert Moelgaard
Senior Consultant
Pharmaceutical Manufacturing Technology & Compliance
Gartnerhaven 18
DK 2800 Lyngby Denmark
T +4553792775






